Acutaas Chemicals
23 July
Acutaas Chemicals (formerly Ami Organics) is a fast-growing manufacturer of pharmaceutical intermediates and specialty chemicals. The company is experiencing rapid growth driven primarily by its pharma CDMO business, as well as the ramp up of its specialty chemicals segment, which includes electrolyte additives and semiconductor chemicals.
Acutaas's business can be categorised into two broad categories: Pharma Intermediates (85% of FY25 revenues) and Specialty Chemicals (15%). Company's portfolio comprises 610+ products and has a diversified customer base of about 600 customers across 55 countries.
Management aims for 25% annual sales growth and targets revenues of ~₹1000cr by FY28 in its pharma cdmo division. The strongest near term growth trigger for Acutaas will be its pharma CDMO contract with Fermion to supply intermediates for darolutamide - a fast growing, blockbuster patented drug for prostate cancer.
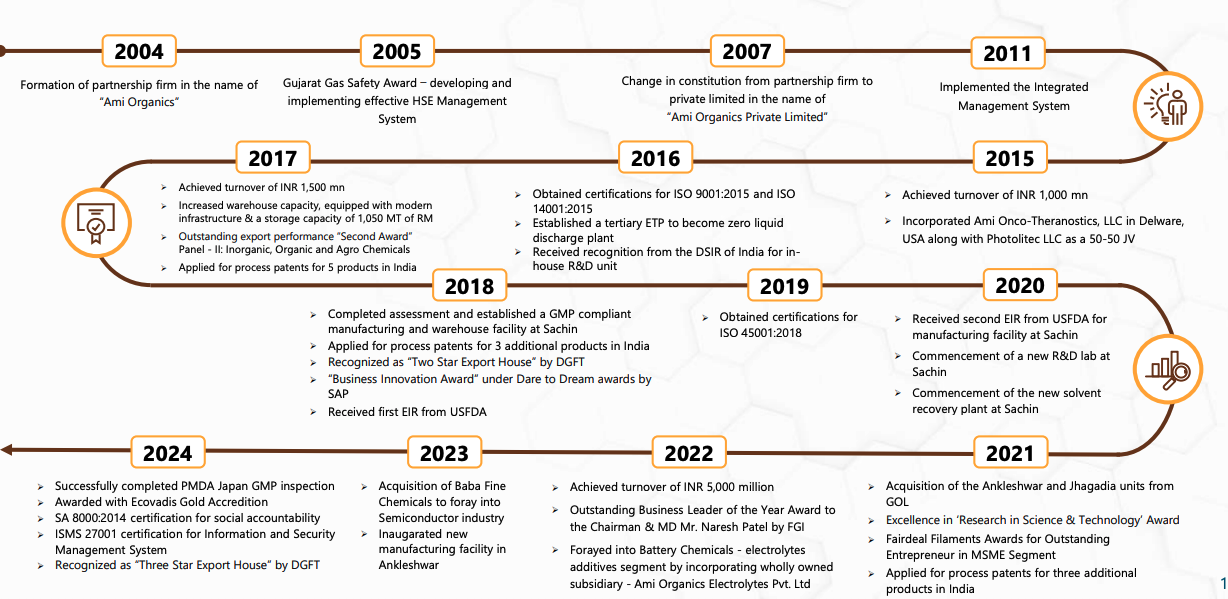
History & Key Milestones
Acutaas Chemicals Limited, was initially formed as a partnership firm under the name "Ami Organics" on January 3, 2004, in Surat by two friends, Nareshkumar Patel and Chetankumar Vaghasia, who had already been business partners for more than two decades - initially in another partnership firm before founding Ami Organics.
Source: Company
Business Segments
Revenue Breakdown by Business Segments (FY25)
85% | ₹854 Cr
15% | ₹152.9 Cr
Revenue Mix by Segment (₹ Cr)
Advanced pharma intermediates accounted for 85% of company's overall revenues in FY2025. The Company develops, manufactures and commercialises intermediates used for manufacturing API and NCE in India and overseas markets in therapeutic areas such as anti-retroviral, anti-inflammatory, anti-psychotic, anti-cancer. Since inception, it has created over 550 pharmaceutical intermediates in 17 different therapeutic areas, which are supplied to over 160 customers. Acutaas has positioned itself as a supplier capable of handling multiple chemistries such as nitration, diazotization, oxidation, esterification, ammoxidation, etc and is highly backward integrated as far as possible for most of its intermediates. As a result company has been able to gain a dominant market share in many of its products.
Key products in Acutaas's pharma intermediates portfolio
| Intermediates | API | Description | Global Market Share |
|---|---|---|---|
1-(3-chlorophenyl)-4-(3-chloropropyl)piperazine hydrochloride 2H-(1,4)-triazole-(4,3-a)pyridine-3-one 1-(3-chlorophenyl)piperazine | Trazodone | Antidepressant that belongs to serotonin receptor antagonists. Used to treat major depressive disorder. | 80-90% |
Methyl-5-acetyl-1H-pyrazole-3-carboxylate 2-chloro-4-(1H-pyrazole-3-yl)benzonitrile Tert-butyl (1S)-2-hydroxy-1-methyl ethyl(carbamate) | Darolutamide | NSAA used to treat nmCRPC. Blocks androgens that promote prostate cancer cell growth. | - |
| 3,4-dihydroxy-5-nitrobenzaldehyde | Entacapone | Used in Parkinson's treatment. Increases levodopa levels to manage wearing-off symptoms. | 80% |
1-(4-amino phenyl)-5,6-dihydro-3-(4-morpholinyl)-(2H)- Ethyl chloro(4-methoxyphenyl)hydrazonoacetate | Apixaban | Anticoagulant used to prevent blood clots. Inhibits factor Xa in clotting process. | 50% |
Benzyl 3-methyl-2-oxo-2,3,10-tetrahydro- 1H-pyrazol-4,5'(4H)-isopyrrolo[2,3-d]quinoxaline-8'(7H)-carboxylate | Lumateperone | Atypical antipsychotic for schizophrenia and bipolar depression treatment. | - |
4-(4-chlorophenyl)piperidin-4-ol 4-(4-aminophenyl)morpholin-3-one | Rivaroxaban | Anticoagulant for blood clot treatment and prevention. Inhibits factor Xa protein. | 50-60% |
| 1-phenylindole | Oxcarbazepine | Used to treat seizure disorders like epilepsy. Decreases nerve impulses causing seizures. | - |
Source: Company, Kotak
As of FY24 company's pharma intermediate business consists of 10% from CDMO, 40% from innovator and 50% from generic pharma intermediates. About 95 % of the portfolio targets fast growing chronic therapy (anticancer, antiviral, CNS and cardiovascular segments) and company is usually first to market with different routes of synthesis and capabilties to supply intermediates from n-1 to n-12 stages . Once a intermediate supplier becomes a registered source in customer's DMF , there is an entry barrier against new entrants. This becomes a source of moat and leads to limited competition and high market share in chosen molecules.
Acutaas has built strong relationships with important customers like Fermion for which it started supplying intermediates since 2010. Company holds 15 process patents to its name and also adopted newer technologies such as flow chemistry and distributed control systems to make its production processes more efficient and competitive.
Acutaas's key customers

Source: Company
key growth drivers in non CDMO business
Apixaban: Apixaban (innovator brand name Eliquis) is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with non-valvular atrial fibrillation. The global market size of the drug is close to US$20 bn, although this will likely decrease after its patent expires in 2026. There are more than 70 manufacturers of the API. AOL has a sizeable market share for two intermediates used to produce the API: 1(4-aminophenyl)-3-morpholin-4-yl-5,6-dihydropyridine-2(1H)-one and ethylchloro [(4- methoxyphenyl) hydrazono] acetate. AOL has already established a healthy pipeline of 25-30 customers for the post-patent era.
Lumateperone tosylate: Lumateperone (innovator brand name Caplyta) is a medication used to manage and treat schizophrenia and other neuropsychiatric disorders. Its patent expiry is 2029. AOL holds a process patent for one of its intermediates: benzyl, 3-methyl-2-oxo-2,3,9,10-tetrahydro-1H-pyrido[3',4':4,5]pyrrolo[1,2,3-de]-quinoxaline-8(7H)-carboxylate.
Rivaroxaban: Rivaroxaban (innovator brand name Xarelto) is an anticoagulant medication used to treat and prevent blood clots. The drug was initially developed by Bayer and marketed by Johnson & Johnson. Its patents are expiring by 2026, and AOL will look to supply four intermediates to generics suppliers.
Trazodone: Trazodone is the largest API in terms of revenues for the company and has been contributing 100cr+ of revenues for the past 2-3 years. AOL offers three intermediates used to make this API and claims to hold a dominant market share (80-90% in FY2021) in these.
CDMO business : key growth driver
In it's cdmo business Acutaas undertakes contract manufacturing for pharma companies under long term supply contracts. Company has commenced a 10 year contract with Fermion Oy of Finland to supply intermediates for darolutamide ( brand name nubeqa which has become a blockbuster for Bayer). This contract will be a major growth driver for Acutaas in coming years . Acutaas is currently supplying 5 intermediates for the molecule Darolutamide. Bayer is selling NUBEQA as the end product to patients/customers. Acutaas is also working on several new CDMO contracts with other pharma innovators with multiple projects in pipeline to be commercialized by FY26.
Prostate Cancer Treatment Market
Prostate cancer is a general term used for diseased condition in which abnormal cells grow and invade in uncontrolled manner in the prostate. Prostate cancer inhibits production of male sex hormones (i.e. androgens) and ultimately blocks the action of androgen.
Global prostate cancer treatment market (2015-25E, US$ bn)
Global prostate cancer treatment market - by drug class
Classification of Hormone Therapies for Prostate Cancer
(Androgen Deprivation Therapy)
Leuprolide
Triptorelin
Relugolix
(Androgen Pathway Inhibitors)
Antiandrogen drugs are medications that block androgens (male sex hormones) from binding with the androgen receptors that exist in prostate cancer cells. In its early stages, prostate cancer cells rely on androgens to feed their growth. By blocking the coupling of androgens with cancer cells, antiandrogens starve the cancer cells of the androgens they need to grow. However, anti-androgens don't stop androgen production. Medical professionals often combine anti-androgens with other treatments, such as surgical or chemical castration.
First-generation ARIs, like Bicalutamide, are the original, weaker drugs that partially block the androgen receptor. Second-generation ARIs (Nubeqa, Xtandi) are the modern standard, providing a much more potent and complete blockade of the receptor for a superior clinical effect.
Across the prostate cancer space, older androgen receptor inhibitors command the highest share. With newer ARi assets Erleada and Nubeqa , despite being third to market, Nubeqa now leads Erleada in every setting , suggesting physician preference for Nubeqa which could be due to the benign safety profile of the drug with similar/ if not better efficacy.
Comparison: Nubeqa vs. Xtandi vs. Erleada ( key ARIs)
| Feature | Nubeqa | Xtandi | Erleada |
|---|---|---|---|
| Primary Advantage | Best-in-class safety profile | Market leader; established efficacy | Strong efficacy |
| Efficacy | Comparable to peers | Comparable to peers | Comparable to peers |
| CNS Side Effects | Lowest Risk | Highest Risk | Intermediate Risk |
| Drug Interactions | Low Potential | High Potential | High Potential |
| Administration | Twice daily with food | Once daily | Once daily |
| 2024 Sales | ~$1.7B | ~$6.6B | ~$3.0B |
| Market Position | Fastest-growing ARI | Market Leader | Strong #2 Competitor |
market share trends in prostate cancer drugs prescription (%)
Notable shifts between Xtandi and Zytiga in mCRPC and limited share for other agents
Source: IQVIA BrandImpact
Key prostate cancer drugs 2024 sales
| Drug (Brand & Generic) | Company / Marketer(s) | 2024 Global Sales (USD $) | Drug Class |
|---|---|---|---|
| Xtandi (Enzalutamide) | Pfizer / Astellas | 6.6 Billion | Androgen Receptor Inhibitor |
| Lynparza (Olaparib) | AstraZeneca / Merck | 4.2 Billion | PARP Inhibitor |
| Erleada (Apalutamide) | Johnson & Johnson | 3.0 Billion | Androgen Receptor Inhibitor |
| Pluvicto (Lutetium Lu 177) | Novartis | 1.8 Billion | Radioligand Therapy |
| Nubeqa (Darolutamide) | Bayer | 1.7 Billion | Androgen Receptor Inhibitor |
| Orgovyx (Relugolix) | Pfizer | 615 Million | GnRH Antagonist |
| Talzenna (Talazoparib Tosylate) | Pfizer | 367 Million | PARP Inhibitor |
| Xofigo (Radium 223 Dichloride) | Bayer | 273 Million | Radiopharmaceutical |
| Akeega (Niraparib / Abiraterone) | Johnson & Johnson | 98 Million | PARP Inhibitor + Antiandrogen |
About Darolutamide
Darolutamide (marketed by Bayer under the brand name Nubeqa) is a drug to combat prostate cancer. It was initially approved in 2019 to treat non-metastatic castration-resistant prostate cancer (nmCRPC), but subsequently in August 2022 also approved by the USFDA in fighting metastatic (i.e., cancer cells that are spreading to other parts of the body) castration-sensitive prostate cancer (mCSPC) in combination with docetaxel. Darolutamide was developed jointly by Bayer and Orion Pharma. Fermion (a fully-owned subsidiary of Orion) manufactures the darolutamide API, while Orion manufactures the formulations and Bayer holds the marketing and distribution rights.
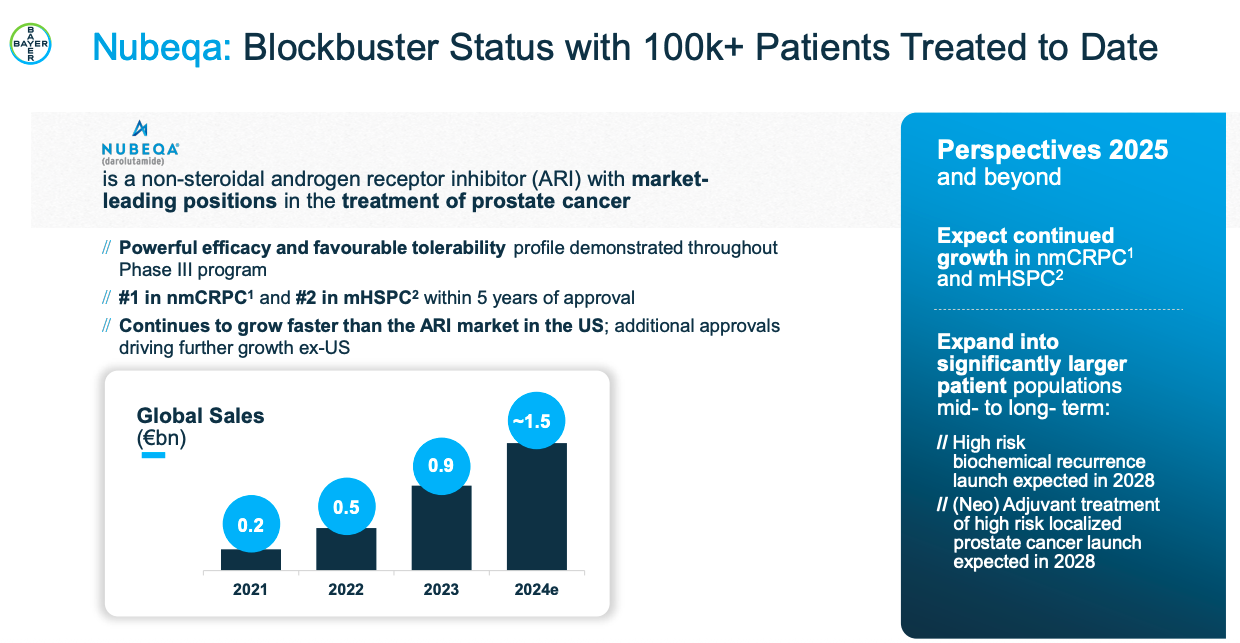
Quarterly sales trend of Nubeqa sales by Bayer ( CY , Euro mn)
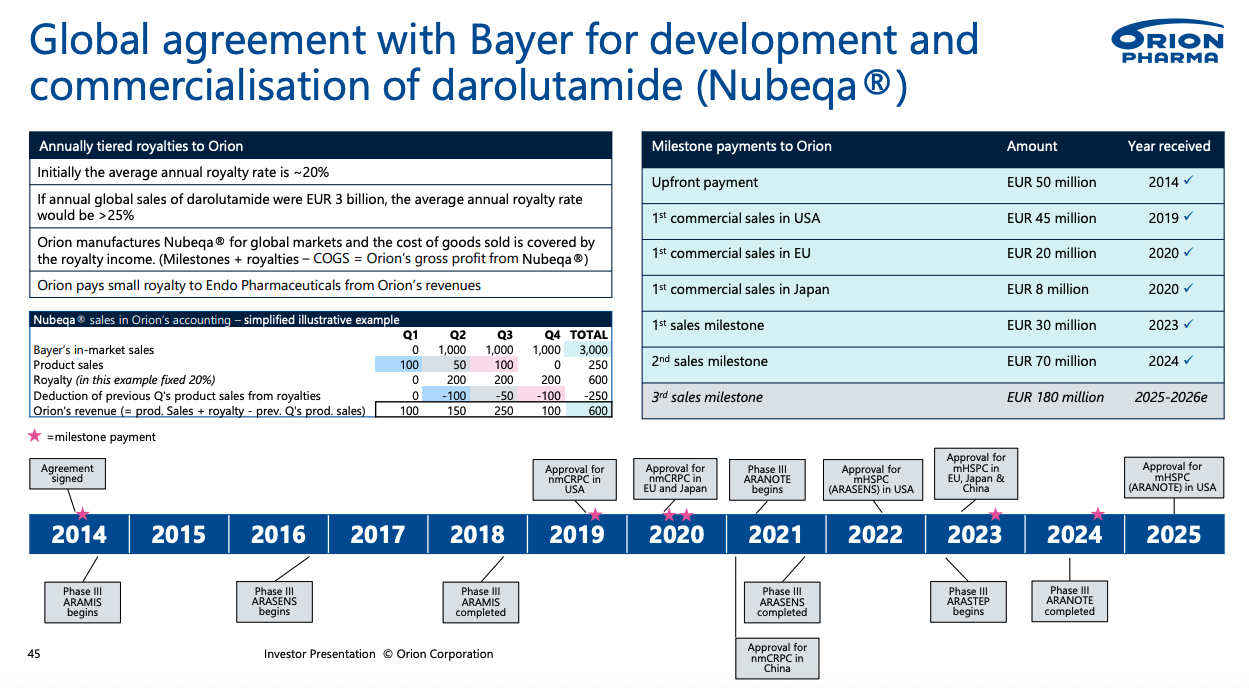
Fermion manufactures the darolutamide API, upon which Orion manufactures the formulations and sells to Bayer at COGS; in exchange, Fermion/Orion receive a combination of royalties and milestone payments. The average annual royalty rate is currently above 20% including product sales to bayer. As the global sales increase, annual royalty rates will increase. If annual sales for darolutamide hits EUR 3 billion, Orion's royalty rate will increase above 25%.
arrangement details between bayer and orion pharma
Source: Company
Darolutamide patent expiry timeline
| Country | Patent Expiry |
|---|---|
| Germany | 2035 |
| France | 2035 |
| Italy | 2035 |
| Switzerland | 2035 |
| Spain | 2035 |
| UK | 2035 |
| China | 2030 |
| Japan | 2035 |
| Brazil | 2030 |
| Canada | 2032 |
| USA | 2033 |
Efficacy of darolutamide versus peer drugs
Studies have shown that darolutamide offers certain advantages over other prostate cancer treatments, particularly in terms of efficacy and safety.
Minimal Blood-Brain Barrier Penetration: Its low penetration of the blood-brain barrier reduces central nervous system-related adverse events such as seizures, fatigue, dizziness, and mental impairment, making it better tolerated compared to other androgen receptor inhibitors.
Efficacy in Prolonging Metastasis-Free Survival: Clinical trials such as ARAMIS demonstrated that darolutamide significantly prolongs metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer, with a median metastasis-free survival of 40.4 months compared to 18.4 months with placebo.
Overall Survival Benefit: Darolutamide significantly reduces the risk of death by approximately 31% compared to placebo.
Favorable Safety Profile: Darolutamide is associated with a low incidence of adverse events, with rates similar to placebo for many side effects including fatigue, falls, fractures, seizures, cognitive disorders, and hypertension.
Better Tolerability: Indirect comparisons suggest darolutamide has a more favorable tolerability profile than apalutamide and enzalutamide, with fewer treatment-emergent adverse events like falls, fractures, rash, fatigue, and mental impairment.
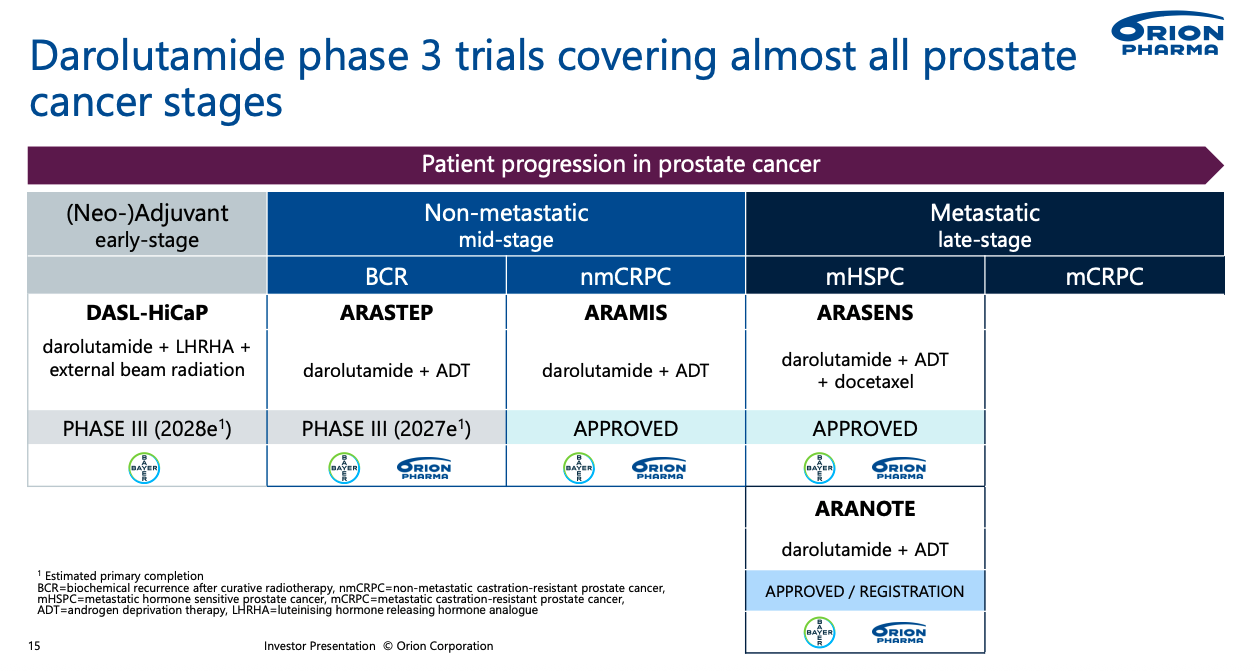
Looking ahead, Orion is conducting two significant clinical trials for darolutamide, named DASL‑HiCaP and ARASTEP.
ARASTEP (phase III, PSMA‑positive biochemical recurrence) - evaluates darolutamide + ADT in men whose PSA is rising after local therapy but before overt metastasis; success could shift Nubeqa into the early‑recurrence setting and extend therapy duration per patient.
DASL‑HiCaP (phase III, adjuvant high‑risk localized PCa) - tests darolutamide as part of adjuvant ADT after surgery or radiotherapy in very‑high‑risk local disease; a positive read‑out would move the drug even earlier in the treatment cascade, opening an entirely new adjuvant market.
Darolutamide opportunity size for Acutaas
We assume that global sales of darolutamide grow to EUR 3.2 bn in CY2028 (from EUR 1.5 bn in CY2024), boosted by the expansion of its use to additional indications. Assuming that the value of the intermediates supplied is 3.5% of final formulation sales, the total addressable market would be EUR 112 mn. Assuming a 50% wallet share for acutaas with fermion , its annual revenue could exceed ~€56 million (~₹570cr at ₹101/€) by CY2028.
CDMO opportunity aside from darolutamide
Darolutamide is only the first product being supplied by Acutaas under the CDMO model; there are several other products currently under discussion with prospective customers. These molecules should begin to contribute to revenues over the next 1-2 years; hence management's guidance for Rs1000cr of revenues from the CDMO business overall (including darolutamide) by FY2028.
Speciality Chemicals
Acutaas began to strategically diversify beyond advanced pharma intermediate in 2021, when it acquired two manufacturing sites (Ankleshwar and Jhagadia) from Gujarat Organics. In 2023, company acquired a 51% stake in Baba Fine Chemicals entering the niche of high-purity semiconductor chemicals. Company also has forayed into electrolyte additives where it will initially start with manufacturing Vinylene carbonate and fluoroethylene carbonate.
Electrolyte additives business
Acutaas has forayed into battery chemicals by setting up production capacities for electrolyte additives under a wholly owned subsidiary named ami organics electronics private limited . Company will have 2000kpta capacity each for vinylene carbonate and fluoroethylene carbonate and expects commissioning of plant by end of h1 fy26 and supplies starting from h2 of fy26. Acutaas has obtained an order from an OEM of a battery cell manufacturer which will take half of acutaas's capacity. These additives are used in small doses within Li-ion battery electrolyte formulations to dramatically improve cell life, safety, and performance.
Acutaas will be the first global manufacturer outside China to develop electrolyte additives (vinylene carbonate and fluoroethylene carbonate) for lithium‑ion batteries; initial capacity of 2,000 MT each and a pipeline of 9+ additives under development. Company has also Signed an MOU with the Gujarat Government for about INR 300cr investment to set up an electrolytes facility. These additives are used in the preparation of electrolyte formulation of Li-ion batteries.
electrolyte additives portfolio
Source: Company
Gujarat Organics business
Acutaas acquired 2 sites at Ankleshwar and Jhagadia from Gujarat Organics in 2021 . Under this business Acutaas manufactures and supplies speciality chemicals and preservatives - including paraben and paraben formulations , methyl salicylate and range of other speciality chemicals used in personal care , pharmaceuticals and agrochemical industries. However, this line of business is largely commoditized with modest margins. Rationale for this acquisition per Acutaas's management was to bag the manufacturing sites . Company does not have any major capex plans in this business and the unutilized land has been converted into a pharma cdmo unit.
Semiconductor Chemicals via Baba fine chemicals
This Segment Includes high‑purity semiconductor chemicals via the Baba Fine Chemicals unit and Acutaas is the only manufacturer in India producing photoresist chemicals domestically. These chemicals are used in semiconductor fabrication in materials for photolithography such as photoresists, anti-reflective coatings, and photo acid generators, as well as precious-metal-based conductive inks (silver, copper, gold, platinum inks) for chip and PCB manufacturing.
Company acquired a majority stake in Baba Fine Chemicals in 2023. BFC was founded by three scientists in 2002 , Before the acquisition by Acutaas , BFC was focused only on 1 single customer - Heraeus Epurio - a German company with operations worldwide . Volumes supplied had been very small and so business was very small at time of acquistion with revenues at 40cr in fy23 with ebitda margins of 65-70%.
Post acquisition, BFC revenues declined in fy24 and fy25 due to supply disruptions with demand weakness in semiconductor industry. Acutaas is actively in discussions with prominent semiconductor materials buyers in korea and japan and expects new orders to materialize by FY26 from these engagements , alongside a potential rebound from the original customer as the chip cycle improves. Furthermore, India's push to establish a domestic semiconductor ecosystem (e.g. new ATMP/OSAT facilities by Micron, government incentives for chip fabs) could create local demand for photoresist and ancillary chemicals in the coming years.
Management guides that the semiconductor chemicals business could scale 2–3x its current sales over the next 2-3 years and it is relatively higher margin business ; so margins for the company will expand as this line of business expands.
BFC's product portfolio
| Product | Description |
|---|---|
| Chloroacetoxy norbornene lactone | Chemical intermediate for organic synthesis and potential drug candidate. Reactive building block for various chemical reactions. |
| PLPM | Crosslinker for organic resins |
| Triphenyl salts (TPS) | Photoacid generators |
| Ultra photoacid generators (ULP) | Photoacid generator sensitive to I-line wavelength of ultraviolet light |
Manufacturing Facilities

Source: Company
Acutaas operates four manufacturing units in India, each supporting different segments of its business. Unit I - Sachin (Surat, Gujarat) is the flagship plant for advanced pharmaceutical intermediates. This facility has an installed reactor capacity of ~144 KL (with 13 production lines and 40 reactors) and is fully cGMP-compliant. Unit I also houses the main R&D center for the company, and it currently operates at around 70–80% capacity utilization.
Unit II – Ankleshwar (Gujarat) was inaugurated in December 2023. The company had acquired an older Ankleshwar facility in 2021 as part of the Gujarat Organics acquisition, but that aging unit was demolished and replaced with this state-of-the-art brownfield plant. The new Ankleshwar unit is spread over ~10,375 m^2 and is equipped with ~90 reactors (~436–442 KL total capacity) along with modern automation (DCS and powder transfer systems). It is designed to cater pharma intermediates and CDMO contracts.
Unit III – Jhagadia (Gujarat) is focused on specialty chemicals production. This facility (acquired from Gujarat Organics in 2021) is much larger in land area (~56,998 m^2) with an installed capacity of about 512 KL. It manufactures products like preservatives and personal care specialty chemicals such as parabens, methyl salicylate and other speciality chemicals. This unit currently runs at roughly 60% utilization and a new electrolyte additives plant is under construction here as a brownfield expansion. Company has ample land here for any further brownfield expansion
Unit IV – Greater Noida (Uttar Pradesh) is a small-scale facility operated via Acutaas's subsidiary Baba Fine Chemicals (BFC) and installed capacity of ~1.8 KL of glass-lined reactor capacity geared towards semiconductor chemicals.
| Plant (Unit) | Location | Capacity (KL) | Land (sqm) | Products / Focus | Certifications / Notes |
|---|---|---|---|---|---|
| Unit I – Sachin | Surat, Gujarat | 144 | 8,250 | Advanced pharma intermediates | GMP-compliant; USFDA & PMDA approved; R&D center |
| Unit II – Ankleshwar | Ankleshwar, Gujarat | 442 | 10,375 | Pharma intermediates (CDMO) | New 2023 plant; 90 reactors; fully automated |
| Unit III – Jhagadia | Jhagadia, Gujarat | 512 | 56,998 | Specialty chemicals | Parabens & fine-chem; battery additives expansion |
| Unit IV – Noida | Greater Noida, UP | 1.8 | 999 | Semiconductor chemicals | Only Indian photoresist source; lab-scale facility |
Key growth triggers
1. CDMO Ramp-up
Management targets ~1000cr revenues from CDMO by FY28 as it ramp up production of intermediates for darolutamide. Along with that company has a strong pipeline of molecules with many molecules in verification phase, which can be an optionality for the company.
2. Patent Expiry Wins
Several core pharmaceutical intermediates are expected to see volume uptick as major drug patents expire in 2025–2026, driving higher demand from generic manufacturers.
3. Ramp up of Speciality Chemicals
Semiconductor chemicals which are currently going through a down cycle, as demand comes back along with the company's initiatives to expand customer base to Taiwan, Korea, Japan can bring new orders as customer qualifications conclude. Battery electrolyte additives are a new growth driver; a dedicated plant (Jhagadia) will be operational by H2 FY26 to start contributing to revenues. Company is positioned as a non china supplier for electrolyte additives. As speciality chemicals segment scales up , margins for the company will expand owing to better mix.
4. Capacity & Efficiency
Major capacity expansion at Ankleshwar (4x reactor increase) completed, ensuring ample headroom for growth. A new pilot plant is being set up to accelerate scale-up of high-potency chemicals and CRAMS projects, enabling faster commercialization of new products. Additionally, in-house solar power (10.8 MW commissioned) will reduce operating costs and support margin improvement as volumes grow.
Key risks
1. Semiconductor Demand Slump
Prolonged weakness in legacy semiconductor markets could delay the recovery and scale-up of Acutaas's photoresist chemicals business (Baba Fine Chemicals), keeping the specialty segment growth muted.
2. Regulatory Compliance
As a chemicals supplier to regulated pharma markets, any lapse in GMP quality standards or environmental/pollution norms could lead to production halts or export restrictions. The business environment is highly regulated, and non-compliance can severely disrupt operations.
3. Tariffs if levied by the US on pharma imports
The US government has launched a investigation into pharma imports, which will likely culminate in tariffs within the next 6-12 months. This could impact demand for some of Acutaas's customers, particularly those supplying generics to the US. It could also impact margins, as it may not be possible to fully pass on heavy tariffs to end-consumers.
4. Inability to secure customer approvals for new products in segments
Regulatory approval processes can be lengthy and uncertain, potentially delaying revenue recognition from new product launches.
5. Seasonality and lumpiness in business
CDMO businesses are generally lumpy and cyclical in nature. For acutaas H1 accounts for 40% of the topline and H2 with 60%. the nature of business is such where nothing happens for decades while months where decades happens. Company has been supplying intermediates to fermion since 2010, building relationships is a moat in this business . However if company fails to win new orders sales might get impacted.
6. Nubeqa's growth slowing down
Any deceleration in Nubeqa's market penetration or competitive pressure from other ARIs could impact Acutaas's key CDMO revenues from darolutamide intermediates.
This post has been provided solely for information purposes and does not constitute an offer or solicitation of an offer or any advice or recommendation to purchase any securities or other financial instruments and may not be construed as such. The author makes no representations as to the accuracy or completeness of any information in this post , Do your own dillegence and consider this blog as just an educational piece.